Here’s A Quick Way To Solve A Info About How To Find Out The Number Of Valence Electrons

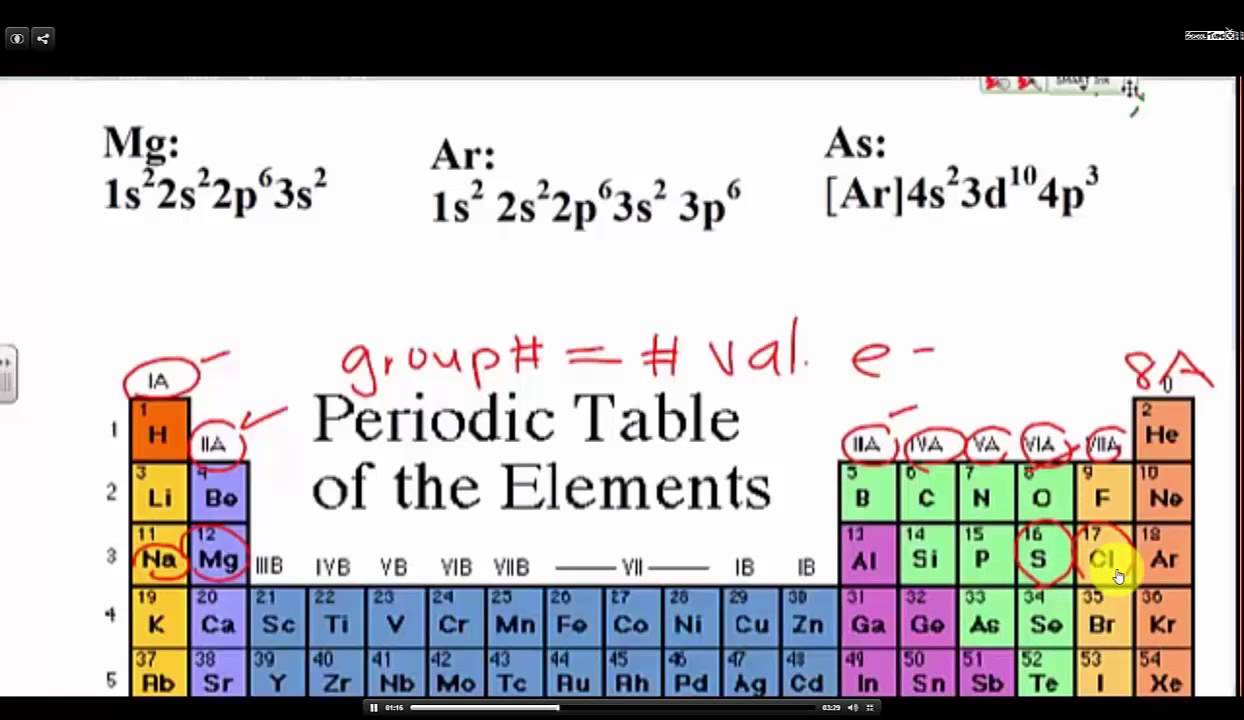
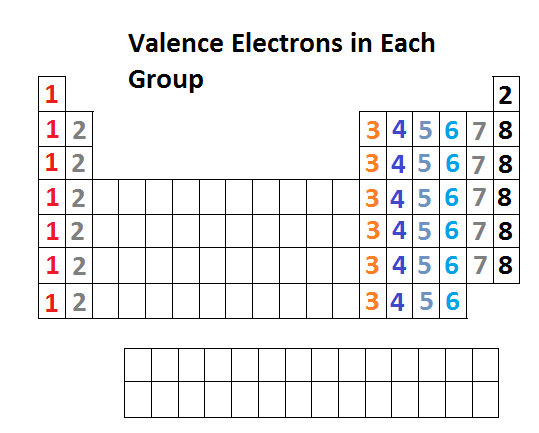
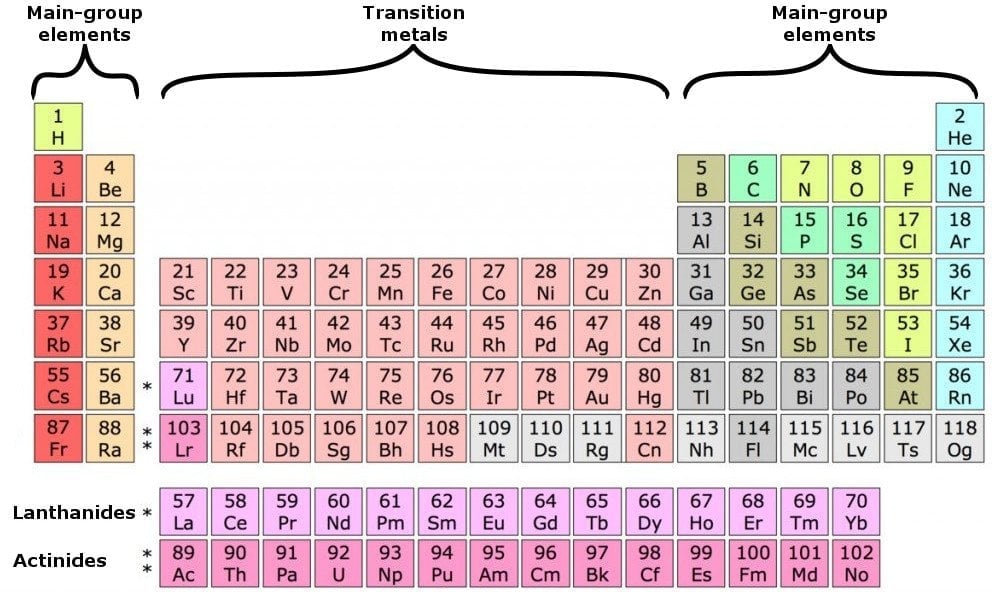
Looking at the periodic table, atoms have a regularly occurring number of valence electrons based on.
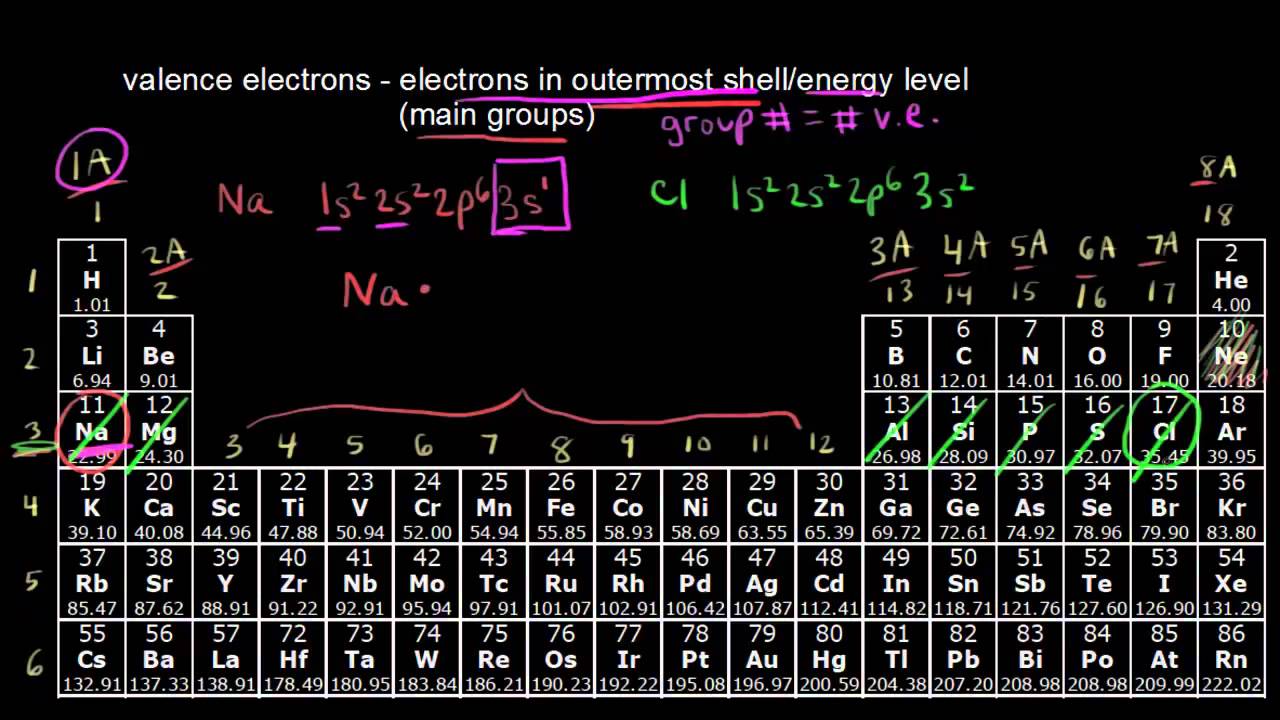
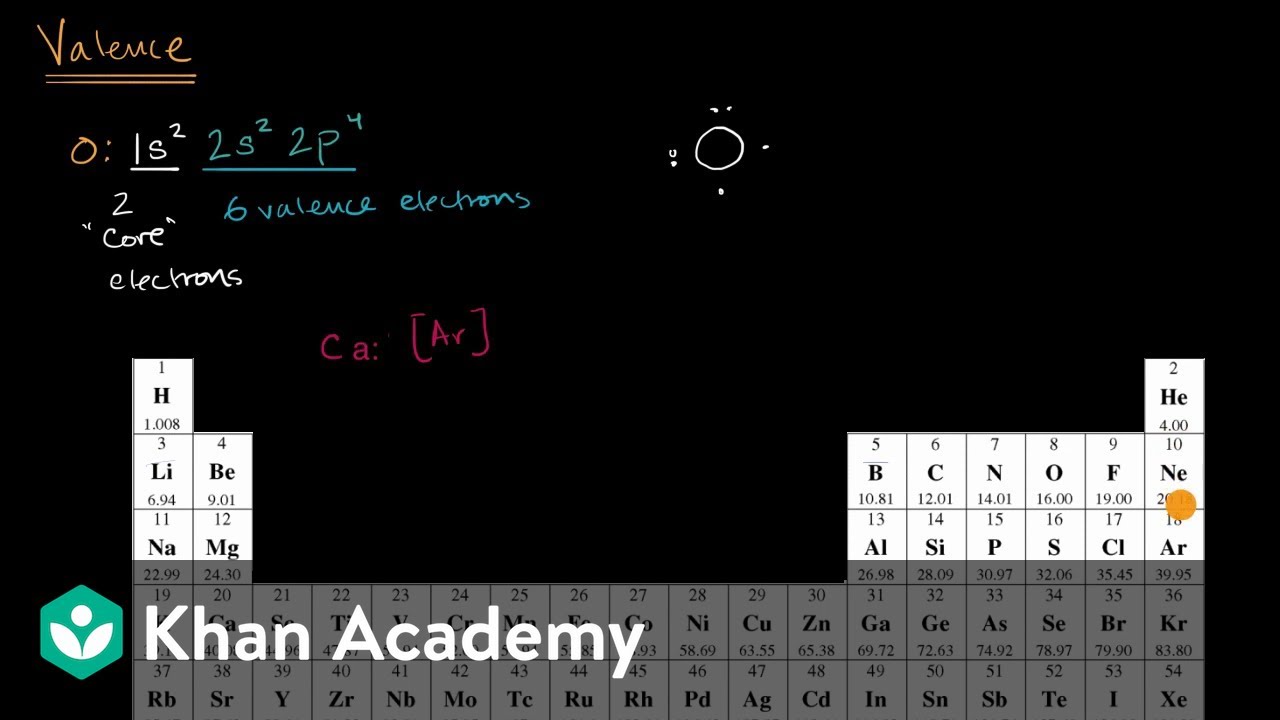
How to find out the number of valence electrons. The first is just to look at the periodic table. Now, we have a total of 6 electrons in the last (3rd) orbital. For fe this means we have 8 valence electrons.
But the valence electrons of the transition elements are located in the inner orbit. For transition metals, like fe,. To find out the valence electrons of phosphorus, you have to see the position of phosphorus in the periodic table.
You can use the periodic table to help you determine the number of valence electrons in an element. The periodic table contains rows and columns. $1s^2, 2s^2, 2p^6, 3s^2, 3p^4$, where the superscripts of each orbital represent the number of electrons in it.
This is a key first step for drawing lewis dot structures for molecules. An explanation of how to find the number of valence electrons for molecules. For the transition element, the valence electrons have to be determined by adding the total electrons.
This includes finding the number of valence electrons for negative and positive. There are two ways to find out the valence electrons of nitrogen. [ar] 3d6 4s2 this allows us to look at the number of electrons outside of the noble gas core.
You are wondering about the question what are valence electrons used for by an element but currently there is no answer, so let kienthuctudonghoa.com summarize and list the top articles. You can see in the electron configuration of iodine ( 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5) that the highest energy level is 5. Nitrogen is a member of group number 15 in the periodic table also.

![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://i.ytimg.com/vi/GEnqFx8MQ5w/maxresdefault.jpg)